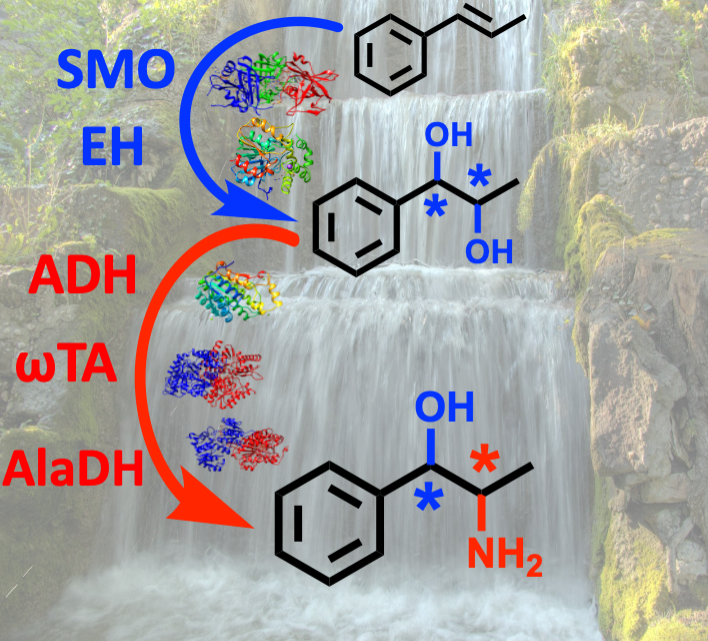
July 2021: Oxidoreductases enable the high regio- and stereoselective synthesis of all phenylpropanolamine stereoisomers from β-methylstyrene
Through the simultaneous combination of stereocomplementary alcohol dehydrogenases and ω-transaminases, we have developed a cascade for the synthesis of all stereoisomers of nor(pseudo)ephedrines with excellent optical purities and high yields. The cascade requires 1-phenylpropane-1,2-diols as key intermediates, which are obtained from β-methylstyrenes by combining a styrene monooxygenase with stereocomplementary epoxide hydrolases.
Abstract
We present a one-pot cascade for the synthesis of phenylpropanolamines (PPAs) in high optical purities (er and dr up to >99.5 %) and analytical yields (up to 95 %) by using 1-phenylpropane-1,2-diols as key intermediates. This bioamination entails the combination of an alcohol dehydrogenase (ADH), an ω-transaminase (ωTA) and an alanine dehydrogenase to create a redox-neutral network, which harnesses the exquisite and complementary regio- and stereo-selectivities of the selected ADHs and ωTAs. The requisite 1-phenylpropane-1,2-diol intermediates were obtained from trans- or cis-β-methylstyrene by combining a styrene monooxygenase with epoxide hydrolases. Furthermore, in selected cases, the envisioned cascade enabled to obtain the structural isomer (1S,2R)-1-amino-1-phenylpropan-2-ol in high optical purity (er and dr >99.5 %). This is the first report on an enzymatic method that enables to obtain all of the four possible PPA stereoisomers in great enantio- and diastereo-selectivity.
Publication details
Maria L. Corrado, Tanja Knaus and Francesco G. Mutti: High Regio- and Stereoselective Multi-enzymatic Synthesis of All Phenylpropanolamine Stereoisomers from β-Methylstyrene. ChemBioChem, 2021, 22, 2345-2350. DOI: 10.1002/cbic.202100123


