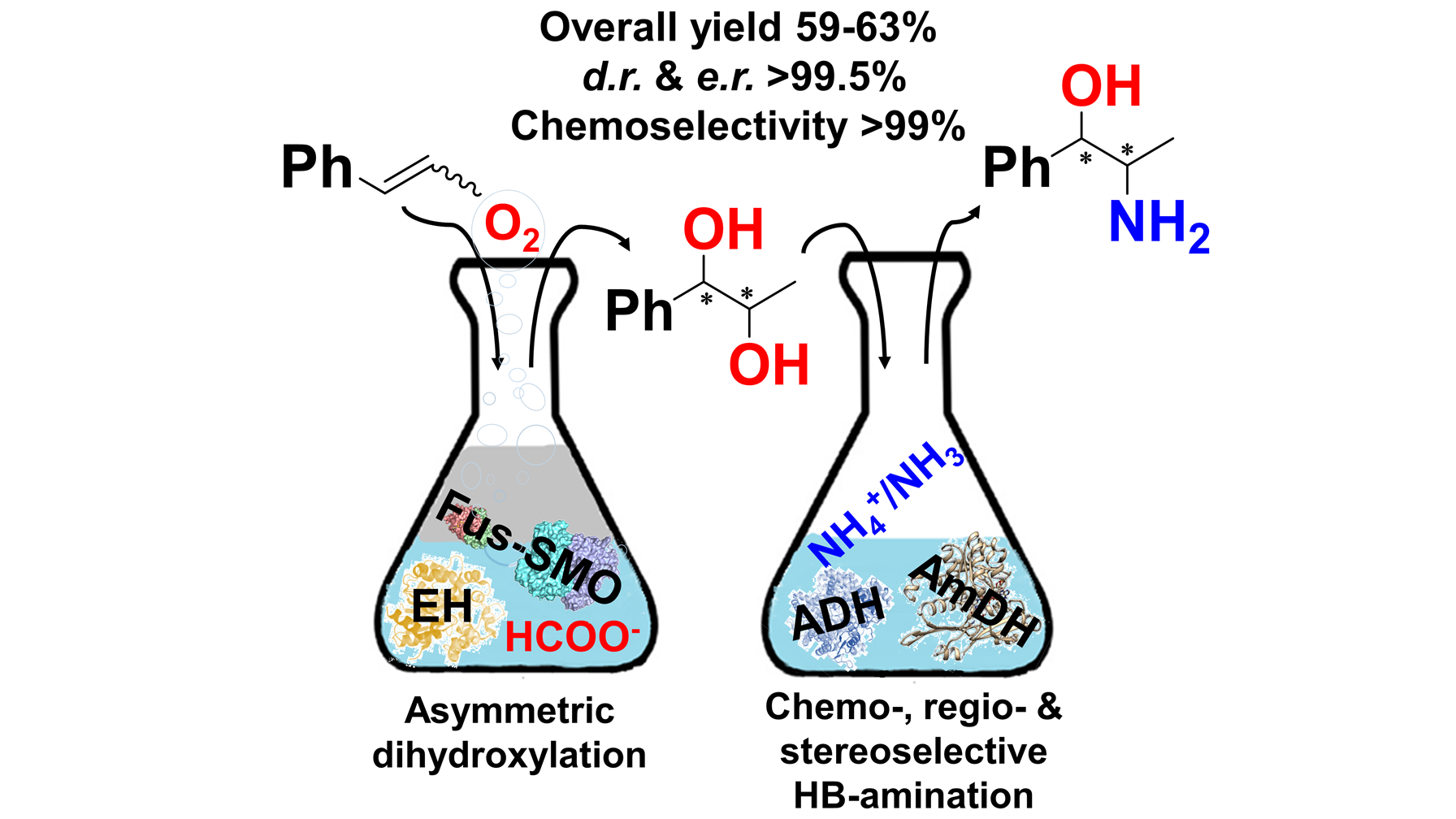
November 2019: Regio- and stereo-selective multi-enzymatic synthesis of chiral phenylpropanolamines
In this work, we have harnessed the potential of the ADH-AmDH (dual-enzyme) hydrogen-borrowing amination for the regio- and stereoselective amination of 1-phenylpropane-1,2-diol (3). Furthermore, the selective alcohol bio-amination was integrated in a multi-enzymatic route that starts from easily available β-methylstyrene substrate (1). This enzymatic route for the formal aminohydroxylation of β-methylstyrene consumes only dioxygen, ammonia and formate, while carbonate is the by-product. The biocascade entails highly selective epoxidation, hydrolysis and hydrogen-borrowing alcohol amination. Thus, β-methylstyrene was converted into 1R,2R and 1S,2R-phenylpropanolamine (5) in 59–63% isolated yields and up to >99.5:<0.5 dr and er.
Corrado, M.L.; Knaus, T.; Mutti, F.G.* Green Chemistry, 2019, 21(23), 6246-6251, DOI: 10.1039/C9GC03161H


